[ad_1]
The preparation of the translation process
In view of the short timeframe for finalisation of the translations and in order to optimise the quality of the translations, the MAHs are strongly advised to prepare for the translation process well in advance in the pre-opinion / position stage, i.e. just following adoption of the PRAC recommendation for variation.
In case of a PSUSA procedure where several MAHs are involved, the EMA will coordinate the translation process by approaching the MAHs individually and provide the timelines accordingly. MAHs should translate all relevant Annexes for each procedure, respectively
Procedures that contain centrally authorised products (CAP(s))
- Annex B: Annexes I, II, IIIA, IIIB, IV1 (scientific conclusions and grounds for the variation of the marketing authorisation) and 127a (risk minimisation measures addressed to Member States)
Procedures that contain a mix of centrally authorised products (CAP(s)) and nationally authorised products (NAP(s))
For the CAP(s):
- Annex B: Annexes I, II, IIIA, IIIB, IV1 (scientific conclusions and grounds for the variation of the marketing authorisation) and 127a (risk minimisation measures addressed to Member States)
For the NAP(s):
- Annex C: it is the CAP MAH’s responsibility to provide NAP Annex C translations
- Annex I (scientific conclusions and grounds for variation to the terms of the marketing authorisations)
- Annex II (amendments to the product information of the nationally authorised medicinal products)
- Annex III (conditions to the marketing authorisations), as applicable
Procedures that contain only nationally authorised products (NAP(s))
- Annex C:
- Annex I (scientific conclusions and grounds for variation to the terms of the marketing authorisations)
- Annex II (amendments to the product information of the nationally authorised medicinal products)
- Annex III (conditions to the marketing authorisations), as applicable
- Annex III or IV (timetable for implementation2), as applicable
During the translation process
Depending on the type of outcome and whether a Commission Decision is required, the timelines for the translation process vary depending on the need for a linguistic review as illustrated below:

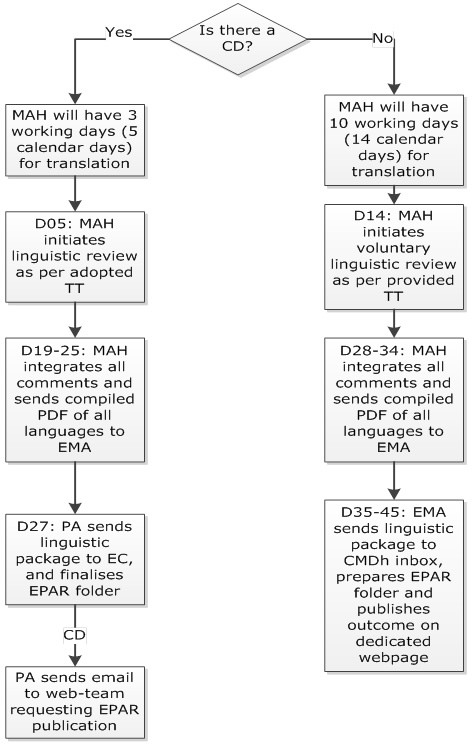
a. In case of CHMP opinion or CMDh position by majority i.e. followed a Commission Decision, the MAH has to provide the translations of the adopted Annexes in all EU languages (including Icelandic and Norwegian – if applicable as detailed below) according to the following timelines:
| Day 5 (5 days after opinion/ position) | Translations of the adopted Annexes in EN and in all other EU languages (including Icelandic and Norwegian) are to be provided electronically (in one Eudralink package if applicable) to the Member States (MS) Contact Points for Translations and to the EMA’s procedure assistant and the PSUSA Mailbox. |
| Day 19 (19 days after opinion/ position) | Member States will send linguistic comments on the Annexes to the MAH by e-mail with a copy to the PSUSA Mailbox. |
| Day 25 (25 days after opinion / position) |
The MAH(s) will implement the required changes, compile the translations and send it back to the EMA. In case of disagreement between a Member State and the MAH, the EMA will not interfere in the translation process at this stage. Disagreements should be solved directly with the concerned MS. In order to facilitate and accelerate the check of the implementation of the’ comments, the MAH should indicate in “QRD Form 2” for each language if all comments have been implemented or not. In the latter case, a justification should be provided for the appropriate language(s) stating why certain comments are not reflected in the final texts. |
b. In a case of CMDh position by consensus, Member States may perform a voluntary linguistic review in the translation process, therefore the following timelines apply:
| Day 1 – 14 (1 to 14 days after position): | MAH translates the adopted Annexes in all other EU languages based on the EN provided version. MAHs with marketing authorisations in Iceland and/or Norway will provide these languages as well. |
| Day 15 (15 days after the position): | Translations of the adopted Annexes in EN and all other EU languages (incl. Icelandic and Norwegian if applicable) are to be provided electronically (in one Eudralink package if applicable) to the Member States (MS) Contact Points for Translations and to the EMA’s procedure assistant and the PSUSA Mailbox for voluntary linguistic check. |
| Day 28-34 (28-34 days after position) |
The MAH(s) will implement the required changes. Translation of the adopted Annexes in EN and in all other EU languages (including Icelandic and Norwegian) are to be compiled and provided electronically (in one Eudralink package if applicable) to the EMA’s procedure assistant and the PSUSA Mailbox. |
| Day 35-45 (35-45 days after position) | The EMA will send the package to the CMDh and prepare the translations for publication. |
In case of an adoption of a European Commission decision addressed to the EU Member States, translation into Irish language is also required since January 2022 but the translation into Gaelic language will be performed by the Translation Centre (CDT) in Luxembourg and reviewed by the Ireland Member State: Product-information requirements.
After the translation process
Once the translations are received from the MAH, the Agency will check if all Member States’ comments have been implemented.
- In case of a CHMP opinion or a CMDh majority position the Agency will compile the Annexes in all languages and send the final copies to the Commission, members of the Standing Committee and the MAH(s) at Day 27 (27 days after opinion).
Following receipt of the final compiled translations, the Commission will start the 22-day Standing Committee consultation, addressing only legal and public health matters (which means in principle no further linguistic review). - In case of a CMDh position (by consensus), the Agency will compile the Annexes in all languages, send the final copies to the Member States and, where applicable, the full set of Annexes will be published on the EMA website.
Standards of translation of Annexes
Please be reminded that in accordance with Union data protection requirements, no personal data should be included in the annotated PIs. This applies to the English version submitted at the time of opinion, the draft translations submitted at D+5 and the final translations submitted at D+25. Please submit annotated PIs in an anonymised format (i.e. names of the reviewers removed from the track-changes). If you do not wish to do so, please ensure that the individuals whose data is included consented to its sharing with EMA and its further sharing by EMA with third parties such as other marketing authorisation applicants, marketing authorisation holders and National Competent Authorities, as relevant. EMA expressly disclaims any liability or accountability for the presence of unnecessary personal data in the annotated PI submitted by the marketing authorisation holder.
- The structure of the English Annexes has to be strictly followed and should be exactly translated as per the adopted English version (i.e. full product information or only amendments to the relevant sections of the product information).
- For translations of Annexes QRD templates for each language should be used
- Make sure that the title pages are adjusted and all brackets (i.e. <>) are deleted from the title.
- Do not leave sections out, do not update the Annex III, e.g. the sections [to be completed on a national level] simply to be translated as ‘to be completed on a national level’.
- Good quality of the translations and compliance with the Member States’ comments is required to facilitate the process.
If a translation is considered not to be of an acceptable quality, the Member State concerned will inform the MAH and the Agency within 3 days of receipt of the translation. The Agency will inform the MAH of the insufficient quality of the translations and the transmission to the Commission will be delayed until receipt of the amended translation (which would be expected within 1 week). A revised timetable will then be prepared.
The MAHs are also strongly advised to liaise directly with the Member States in case of disagreement with any of the comments made or in case further clarification on some comments is required, and to reflect the outcome in “QRD Form 2”.
In addition, the MAHs are reminded that in case the complete product information is part of the Annex III, it should be presented in strict compliance with the QRD Convention (e.g. format, layout and margins).
The Agency will monitor the quality of the translations, the review by the Member States and industry’s compliance with the Member States’ comments as part of the Performance Indicators.
References
1Annex IV are part of the next EPAR publication. However, they will not remain part of the EPAR and will become obsolete with the next following EPAR revision. They, however, remain part of the Commission Decision in the Union Registry on the Commission’s webpage.
2This time table is adopted in case a CMDh position reached by consensus and therefore not followed by a Commission Decision; in case of a majority position, the deadlines foreseen in the legislation for implementation after the Commission Decision apply.
[ad_2]
Source link