[ad_1]
Ten new medicines recommended for approval
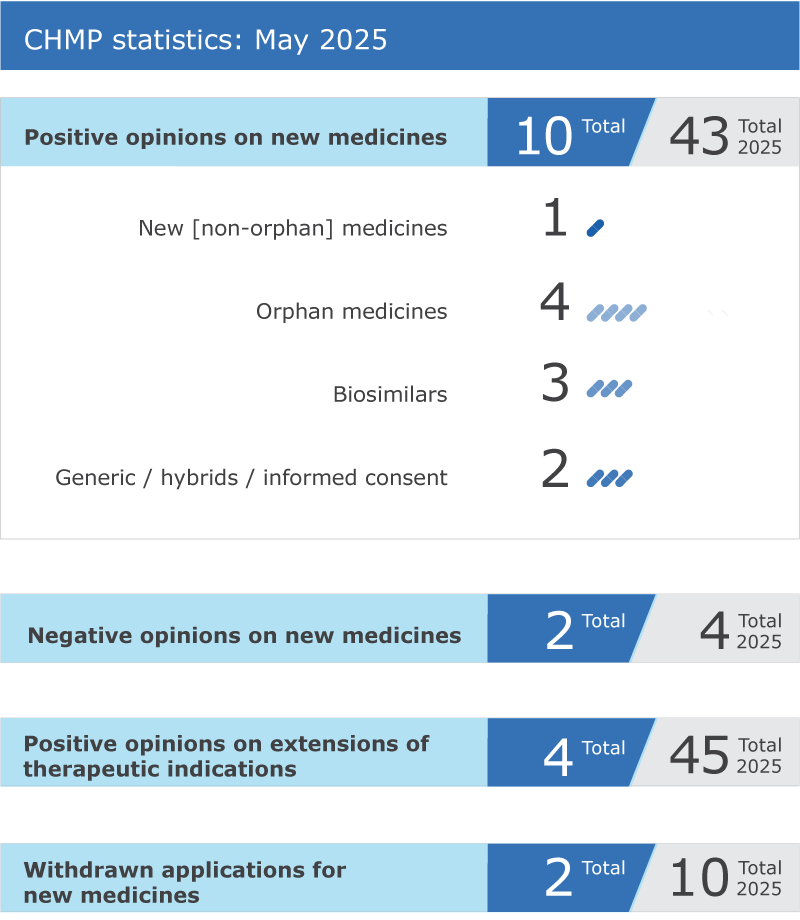
EMA’s human medicines committee (CHMP) recommended ten medicines for approval at its May 2025 meeting.
The committee recommended granting a conditional marketing authorisation for Aucatzyl* (obecabtagene autoleucel), for the treatment of relapsed or refractory B-cell precursor acute lymphoblastic leukaemia, a type of cancer of the white blood cells. This medicine was supported through EMA’s PRIority MEdicines (PRIME) scheme, which provides early and enhanced scientific and regulatory support for promising medicines with a potential to address unmet medical needs. See more details in the news announcement in the grid below.
A positive opinion was adopted for Blenrep* (belantamab mafodotin), for the treatment of relapsed or refractory multiple myeloma, a rare and incurable disease of the plasma cells, which typically affects adults from 60 years of age.
The committee recommended granting a conditional marketing authorisation for Ezmekly* (mirdametinib), for the treatment of paediatric and adult patients with neurofibromatosis type 1, an inherited disease in which the patient develops benign (non-cancerous) tumours along the nerves.
Itovebi (inavolisib) received a positive opinion from the CHMP for the treatment of adults with PIK3CA-mutated, oestrogen receptor (ER)-positive, HER2-negative locally advanced and metastatic breast cancer.
The CHMP adopted a positive opinion under exceptional circumstances for Maapliv* (amino acids), for the treatment of maple syrup urine disease (MSUD) in patients from birth. MSUD is a rare genetic disorder where the body cannot break down certain amino acids found in proteins. This leads to a buildup of harmful substances in the blood and urine, which can cause serious health problems.
The CHMP recommended granting a marketing authorisation for Riulvy (tegomil fumarate), for the treatment of adults and children from 13 years of age with relapsing remitting multiple sclerosis, a disease of the brain and spinal cord in which inflammation destroys the protective covering around nerves and the nerves themselves. This medicine was submitted in a hybrid application, which relies in part on the results of pre-clinical tests and clinical trials of an already-authorised reference product and in part on new data.
The committee adopted positive opinions for three biosimilar medicines:
- Bomyntra (denosumab), for the prevention of skeletal related events in adults with advanced malignancies involving bone and the treatment of adults and skeletally mature adolescents with giant cell tumour of bone.
- Conexxence (denosumab), for the treatment of osteoporosis, a disease that makes bones fragile, in postmenopausal women and in men at increased risk of fractures.
- Rolcya (denosumab), for the treatment of osteoporosis and bone loss.
A generic medicine, Emtricitabine/Tenofovir alafenamide Viatris (emtricitabine / tenofovir alafenamide), received a positive opinion for the treatment of adults and adolescents infected with human immunodeficiency virus type 1 (HIV-1).
Negative opinion for two medicines
The committee recommended not granting a paediatric-use marketing authorisation for Atropine sulfate FGK (atropine), a medicine intended for treating myopia in children aged three years and older.
Kinselby* (resminostat) received a negative opinion from the CHMP for the treatment of patients with advanced stage mycosis fungoides and Sezary syndrome, two cancers of blood cells that affect mainly the skin.
For more information on these negative opinions, see the question-and-answer documents in the grid below.
Recommendations on extensions of therapeutic indication for four medicines
The committee recommended extensions of indication for four medicines that are already authorised in the European Union (EU): Imfinzi, Rezolsta, Saxenda and Tevimbra.
Re-examination of recommendations
On 28 June 2024, the European Commission revoked the refusal of a marketing authorisation for Aplidin (plitidepsin), a medicine expected to be used to treat multiple myeloma. Following the adoption of this decision, the Commission has requested EMA to re-start the re-examination of the negative opinion adopted by the CHMP in December 2017.
The marketing authorisation holder for Winlevi (clascoterone), a medicine intended for treating acne vulgaris, has requested a re-examination of the negative opinion adopted during the committee’s April 2025 meeting.
Upon receipt of the grounds of these requests, the CHMP will re-examine its opinions and issue final recommendations.
Withdrawal of applications
An application for an initial marketing authorisation was withdrawn. Teriparatide Ascend (teriparatide) was intended for the treatment of osteoporosis.
An application to extend the therapeutic indication of Lutathera (lutetium (177Lu) oxodotreotide) in the treatment of adults with newly diagnosed tumours in the gut, known as gastroenteropancreatic neuroendocrine tumours, was also withdrawn.
Question-and-answer documents on the withdrawals of these medicines are available in the grid below.
Conclusion of referral
The committee finalised its review of the use of azithromycin, an antibiotic that has been used for decades to treat a wide range of infectious diseases. The CHMP has recommended several changes, including revisions and removals of certain indications. These recommendations aim to optimise the use of this common antibiotic and minimise the development of antimicrobial resistance, the ability of microorganisms to become resistant to antimicrobials.
For more information on this recommendation see the public health communication in the grid below.
Start of referral
The CHMP started a review of all available information on the benefits and risks of medicines containing ipidacrine. These medicines have been authorised in several EU countries through national procedures and are used in adults to treat different conditions affecting the nervous system. The review of ipidacrine-containing medicines has been initiated at the request of the Irish medicines regulatory agency, under Article 31 of Directive 2001/83/EC.
For more information, see the public health communication in the grid below.
Agenda and minutes
The agenda of the May 2025 CHMP meeting is published on EMA’s website. Minutes of the meeting will be published in the coming weeks.
CHMP statistics
Key figures from the May 2025 CHMP meeting are represented in the graphic below.
*This product was designated as an orphan medicine during its development. Orphan designations are reviewed by EMA’s Committee for Orphan Medicinal Products (COMP) at the time of approval to determine whether the information available to date allows maintaining the medicine’s orphan status and granting the medicine ten years of market exclusivity.
[ad_2]
Source link