[ad_1]
Four new medicines recommended for approval
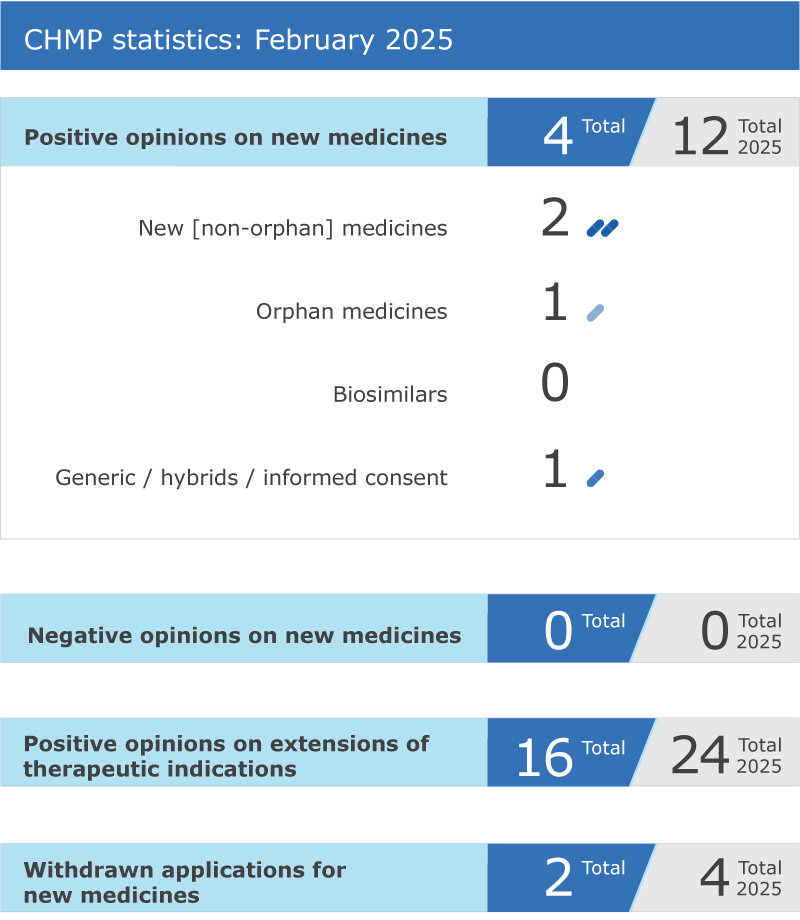
EMA’s human medicines committee (CHMP) recommended four medicines for approval at its February 2025 meeting.
The committee recommended granting a marketing authorisation for Deqsiga (human normal immunoglobulin), intended for replacement therapy in people with primary or secondary immunodeficiencies and immunomodulation in people with certain autoimmune diseases.
The CHMP recommended granting a conditional marketing authorisation for Lynozyfic (linvoseltamab) for the treatment of patients with relapsed and refractory multiple myeloma, a cancer of the bone marrow.
Vyjuvek* (beremagene geperpavec) received a positive opinion to treat wounds in patients of all ages with dystrophic epidermolysis bullosa, a serious, ultra-rare genetic skin blistering disease caused by mutations in the collagen type VII alpha 1 chain (COL7A1) gene. Vyjuvek is expected to bring substantial therapeutic benefits and improve the quality of life for patients with this skin disorder. This medicine was supported through EMA’s PRIority MEdicines (PRIME) scheme, which provides early and enhanced scientific and regulatory support for promising medicines with a potential to address unmet medical needs. See more details in the news announcement in the grid below.
A generic medicine, Trabectedin Accord (trabectedin), received a positive opinion for the treatment of advanced soft tissue sarcoma and of relapsed platinum-sensitive ovarian cancer.
Recommendations on extensions of therapeutic indication for 16 medicines
The committee recommended extending the therapeutic indication of two cystic fibrosis medicines, Kaftrio (ivacaftor/tezacaftor/elexacaftor)and Kalydeco (ivacaftor), to include their use in combination in patients aged two years and older who have at least one non-class I mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. Cystic fibrosis is an inherited disease that has severe effects on the lungs, the digestive system and other organs. This positive opinion brings disease-modifying treatment to all cystic fibrosis patients with modulator responsive mutations. See more details in the news announcement in the grid below.
The CHMP also adopted an extension to the existing indication of the chikungunya vaccine Ixchiq (chikungunya vaccine (live)) to include the active immunisation of adolescents from 12 years of age. This vaccine was initially approved to protect adults against the disease caused by Chikungunya virus. Chikungunya is a viral disease transmitted to humans by infected mosquitoes. See more details in the grid below.
Fabhalta* (iptacopan) received a positive opinion for an extension of indication for the treatment of adult patients with complement 3 glomerulopathy, an ultra-rare kidney disease that previously had no treatment options. See more details in the grid below.
The committee recommended another 12 extensions of indication for medicines that are already authorised in the EU: Abrysvo, Calquence, Columvi*, Darzalex*, Enhertu, Imfinzi, Jaypirca, Prevymis*, Rinvoq, Stelara, Supemtek Tetra and Tremfya.
Outcome of re-examinations
After a re-examination of its initial opinion at the request of the applicant, the CHMP confirmed its initial positive recommendation for an extension of indication for Keytruda (pembrolizumab) to patients with unresectable non-epithelioid malignant pleural mesothelioma. See more details in the grid below.
Following a re-examination, the CHMP also confirmed its initial recommendation to refuse the marketing authorisation for Kizfizo* (temozolomide), a medicine intended for the treatment of neuroblastoma, a rare cancer that forms from immature nerve cells.
For more information on the refusal of this marketing authorisation, see the question-and-answer document in the grid below.
Withdrawal of applications
Applications for an initial marketing authorisation for two medicines were withdrawn:
- Pelgraz Paediatric (pegfilgrastim) was intended to treat neutropenia (low levels of neutrophils, a type of white blood cell that helps to fight infections) and prevent febrile neutropenia (neutropenia accompanied by fever) in children with cancer;
- Rilonacept FGK Representative Service GmbH* (rilonacept) was intended for the treatment of adults and children from 12 years of age with idiopathic pericarditis (inflammation of the membrane around the heart) which keeps coming back.
The application to extend the therapeutic indication of Dupixent in the treatment of moderate-to-severe chronic spontaneous urticaria (itchy rash) in adults and adolescents aged 12 years and older was withdrawn.
The marketing authorisation holder for Bimervax (COVID-19 vaccine (recombinant, adjuvanted)) withdrew its application to include an adapted version targeting the JN.1 strain of SARS CoV-2, the virus that causes COVID-19.
Question-and-answer documents on the withdrawals of these medicines are available in the grid below.
Other updates
The CHMP concluded that its opinion recommending a marketing authorisation for Leqembi (lecanemab) does not need to be updated. In November 2024, Leqembi received a positive opinion for the treatment of mild cognitive impairment (memory and thinking problems) or mild dementia due to Alzheimer’s disease (early Alzheimer’s disease) in patients who have only one or no copy of ApoE4, a certain form of the gene for the protein apolipoprotein E. In January 2025, the European Commission (EC) asked the committee to consider information on the safety of Leqembi that became available after the adoption of the positive opinion and whether this would require an update of their recommendation. The CHMP has now concluded that its opinion recommending the marketing authorisation does not need to be updated and has provided a response to the EC, which will now resume the decision-making process for this medicine. More information is available below.
The CHMP adopted a positive opinion approving changes to the manufacturing process for Champix (varenicline), a medicine used in adults to help them stop smoking. These changes ensure that the presence of a nitrosamine impurity stays below the acceptable intake limit during manufacture and throughout the product’s shelf-life. Further information is available in the ‘Other updates’ section.
Agenda and minutes
The agenda of the February 2025 CHMP meeting is published on EMA’s website. Minutes of the meeting will be published in the coming weeks.
CHMP statistics
Key figures from the February 2025 CHMP meeting are represented in the graphic below.
*This product was designated as an orphan medicine during its development. Orphan designations are reviewed by EMA’s Committee for Orphan Medicinal Products (COMP) at the time of approval to determine whether the information available to date allows maintaining the medicine’s orphan status and granting the medicine ten years of market exclusivity.
[ad_2]
Source link